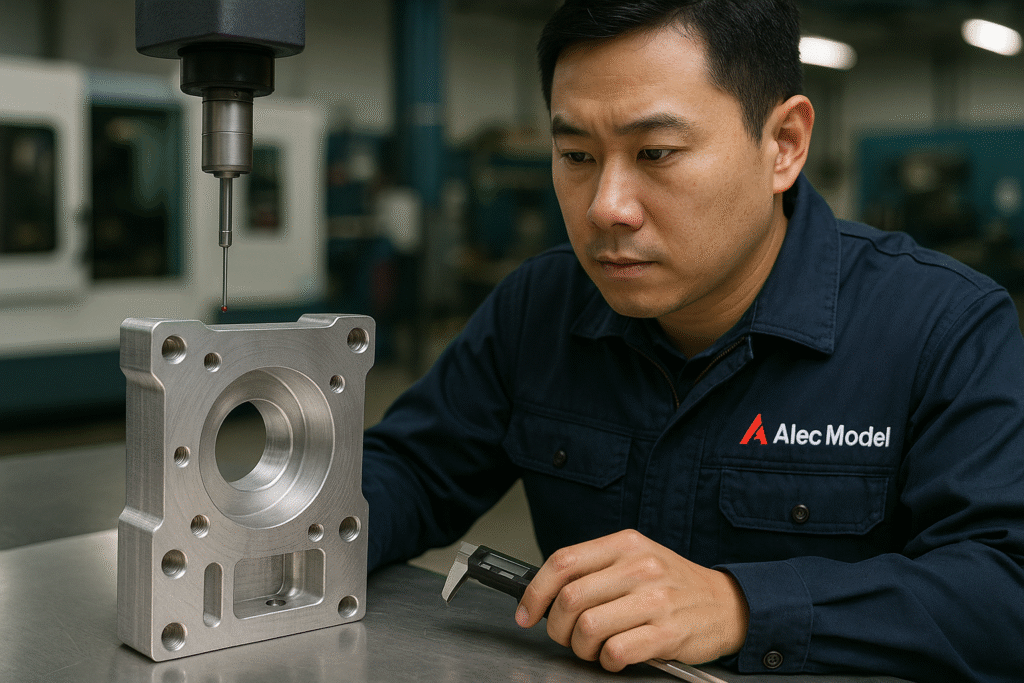
In mid-2024, we received an inquiry from a North American medical device manufacturer. This client specializes in the development and production of high-end orthopedic surgical instruments, primarily exporting to Europe and North America. They have extremely high standards for machining precision, material control, and quality systems.
The client discovered us via LinkedIn and, after learning about our capabilities in precision machining and quality control, formally expressed interest in cooperation.
The parts in this project were to be used in a new type of surgical guide for joint reconstruction. They required extremely high precision, surface treatment, and material performance stability.